Glycation
is a post-translational modification of proteins involving a covalent
linkage between a reducing sugar, such as glucose, and a receptive amino
group via the non-enzymatic Maillard reaction.
During the early stages of the Maillard reaction, the aldehyde
groups of the reducing sugars react with the amino groups of N-terminal
amino acids to form Schiff bases, which are then converted to Amadori
compounds.
In the advanced stages, these Amadori compounds undergo
oxidation, dehydration and condensation to form advanced glycation
end-products (AGE) that are characterized by fluorescence, browning, and
intra- or inter-molecular cross-linking properties. The main AGE products are pentosine and carboxymethyllysine (CML).
Pentosidine
is a main AGE compound that is formed from cross-linked lysine and
arginine residues of proteins and reducing sugars.
Pentosine is a stable AGE-structure and its levels increase in
aging, diabetes, cataracts, renal diseases, chronic rheumatoid
arthritis, photoaged skin and in brain lesions associated with
Alzheimer’s disease.
CML is formed by the irreversible oxidation of Amodori products
and is the major non-fluorescent AGE in uracemia.
AGE formation is especially predominant under conditions of high
sugar concentrations, such as diabetes.
AGEs are thought to play an important role in the structural and
functional alterations that occur in proteins during long-term aging and
diabetes such as blindness, renal failure, neuropathy and vascular
disease.
It
is known that glycoaldehyde is an efficient precursor of CML.
Glycoaldehyde modified proteins have also been shown to contain
high levels of pentosine formation¸suggesting that glycoaldehyde is a
potent precursor of pentosine.
Therefore, glycoaldehyde-modified proteins contain both pentosine
and CML epitopes.
Antibodies raised to glycoaldehyde-modified proteins have been
shown to detect AGE-modified proteins as well as both pentosine-modifed
BSA and CML-modified BSA.
A
goat anti-AGE antiserum produced using a glycoaldehyde-modified
protein is currently available. This antiserum has been shown to
be immunoreactive with glycoaldehyde-modified
BSA by ELISA and detects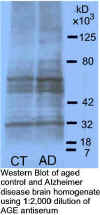 AGE-modified proteins on western blots.
This antibody should be a valuable tool for scientists working to
understand the role of AGE in diabetes, aging and other neurological
diseases.
AGE-modified proteins on western blots.
This antibody should be a valuable tool for scientists working to
understand the role of AGE in diabetes, aging and other neurological
diseases.
Glycoaldehyde-modified
bovine serum albumin is available (Cat
No. HMP036H), which can be used to neutralize immunoreactivity.
Manufacturing
Reference:
Farboud,
B., Aotaki-Keen, A., Miyata, T., et al.
Mol. Vis. 1999; 5:11 (www.molvis.org/molvis/v5/p11).