The
amyloid precursor protein (APP) is a large membrane protein whose C-terminus
projects into the extracellular space. In Alzheimer's disease (AD), the
APP is proteolytically cleaved at the N-terminal of Ab
by b-secretase
(BACE) to release a ~100 kD APPsb protein into the extracellular space.
The remaining 12 kD fragment remains membrane bound where it can be
cleaved at its C-terminus by g-secretase
(presenilins) to release the insoluble Ab
peptide into the extracellular space with the ~8 kD APP C-terminal
fragment (CTFb)
remaining membrane bound. The
APP is the subject of intensive investigations to determine how this
protein is broken down abnormally in AD brains to give rise to Ab,
which is present in senile plaques and vessels.
A goat antiserum to a synthetic peptide that corresponds to
amino acids 681-695 of the C-terminus of human APP is currently available.
This antiserum has been shown to be immunoreactive with the unconjugated
immunizing peptide by ELISA. In western blots it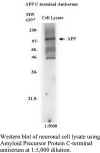 immunolabels the APP holoprotein. The smaller CTFb is rapidly degraded and not detected on westerns.
This antibody should be a valuable tool for scientists working to
understand the role of in Alzheimer’s disease.
immunolabels the APP holoprotein. The smaller CTFb is rapidly degraded and not detected on westerns.
This antibody should be a valuable tool for scientists working to
understand the role of in Alzheimer’s disease.
This
antiserum was produced using proprietary methodology whereby the peptide
is attached to a carrier that elicits minimal immunoreactivity so that the
antiserum has a higher degree of specificity for the peptide.
Since there is no overwhelming production of interfering antibodies
to the carrier, this antiserum can routinely be used without further
purification. Pseud-Immune™ control
immune serum (Cat no. GPA018E) from a mock immunized animal is available
to be used in conjunction with this antibody as well as the immunizing
peptide (Cat no. HSP010C), which can be used to neutralize
immunoreactivity.
Manufacturing
Reference:
Selkoe, DJ, et al.
Proc. Natl. Acad. Sci. USA 85: 7341-7345, 1988.
 Polyclonal Anti-human
Polyclonal Anti-human