The
amyloid precursor protein (APP) is a large membrane protein whose N-terminus
projects into the extracellular space. In Alzheimer's disease (AD), this
protein is broken down and releases the Ab peptide that is found in senile plaques and vessels.
A frameshift mutant of APP has been found that results from a GA
dinucleotide deletion which results in a frameshift mutation that gives
rise to a truncated APP with a unique C-terminus.
The role of the amyloid precursor protein frameshift mutant (APPFM)
in AD is currently unknown. These abnormal proteins may accumulate and
lead to cellular disturbances and neuronal degeneration.
A goat antiserum to a synthetic peptide that corresponds to
amino acids 339-348 of the C-terminus of human APPFM is currently
available. This antiserum has been shown to be immunoreactive with the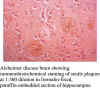 unconjugated immunizing peptide by ELISA. The antiserum will immunolabel
dystrophic neurites in senile plaques and with neurons containing the
APPFM in formalin-fixed, paraffin-embedded sections from AD brain.
It also immunolabels a 45 kD band on western blots that corresponds
to the expected molecular weight of the APPFM protein based on the amino
acid sequence. This antibody
should be a valuable
unconjugated immunizing peptide by ELISA. The antiserum will immunolabel
dystrophic neurites in senile plaques and with neurons containing the
APPFM in formalin-fixed, paraffin-embedded sections from AD brain.
It also immunolabels a 45 kD band on western blots that corresponds
to the expected molecular weight of the APPFM protein based on the amino
acid sequence. This antibody
should be a valuable 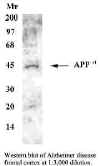 tool for scientists working to understand the role of
APPFM in AD.
tool for scientists working to understand the role of
APPFM in AD.
This antiserum was produced using proprietary
methodology whereby the peptide is attached to a carrier that elicits
minimal immunoreactivity so that the antiserum has a higher degree of
specificity for the peptide. Since
there is no overwhelming production of interfering antibodies to the
carrier, this antiserum can routinely be used without further
purification. Pseud-Immune™
control
immune serum (Cat no. GPA018E) from a mock immunized animal is available
to be used in conjunction with this antibody as well as the immunizing
peptide (Cat no. HSP011C), which can be used to neutralize
immunoreactivity.
Manufacturing
Reference:
Van Leewen, FW, et al.
Science 279:242-247, 1998.
 Polyclonal Anti-human
Polyclonal Anti-human