The
prion protein is a large membrane protein that occurs normally in neurons
of the human brain and is thought to be involved in synaptic transmission.
In prion diseases, such as Creutzfeld-Jakob disease (CJD),
Gerstmann-Straussler-Scheinker syndrome (GSS), Fatal Familial Insomnia (FFI),
Alpers Syndrome and Kuru, the normal cellular form of this protein (PrPc)
is transformed into an altered protein when it comes into contact with an
infectious prion protein (PrPsc) from another host. This altered PrPsc
accumulates in cytoplasmic vesicles of diseased individuals forming
lesions, vacuoles and amyloid deposits.
PrPsc
is a proteolytic-resistant form of PrPc, although no major chemical
differences between the two have been found. The major difference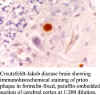 between
the two is that the infectious form has assumed a different conformational
3-D structure. The PrPc protein is highly conserved across many species,
including humans, sheep, mice, hamsters, Drosophila and bovine.
between
the two is that the infectious form has assumed a different conformational
3-D structure. The PrPc protein is highly conserved across many species,
including humans, sheep, mice, hamsters, Drosophila and bovine.
The
prion protein has received considerable attention in the last few years
because it is the same protein that is responsible for bovine spongiform
encephalopathy or "Mad Cow disease" and also scrapie in sheep.
Prion disease can either occur spontaneously by means of  coming in contact
with the infectious agent, such as the outbreak in England with the
infection of several people who consumed contaminated beef, or it can
occur as a rare hereditary form. Currently, there is no cure for prion
diseases and death usually occurs within one year of the onset of
symptoms, which usually include dementia.
coming in contact
with the infectious agent, such as the outbreak in England with the
infection of several people who consumed contaminated beef, or it can
occur as a rare hereditary form. Currently, there is no cure for prion
diseases and death usually occurs within one year of the onset of
symptoms, which usually include dementia.
A
goat antiserum to a synthetic peptide that corresponds to amino acids
79-97 of the N-terminus of the human prion protein PrP27-30 is
currently available. This
antiserum has been shown to be immunoreactive with the unconjugated
immunizing peptide by ELISA. The antiserum will immunolabel amyloid
plaques in formalin-fixed, paraffin-embedded sections from CJD
brain. Western blot analysis
shows that this antibody stains the 29-35 kD glycosylation-depedent prions
in brains of humans, cattle, sheep and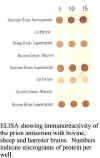 hamsters.
This antibody should be a valuable tool for scientists working to
understand the role of prions in spongiform diseases.
hamsters.
This antibody should be a valuable tool for scientists working to
understand the role of prions in spongiform diseases.
This
antiserum was produced using proprietary methodology whereby the peptide
is attached to a carrier that elicits minimal immunoreactivity so that the
antiserum has a higher degree of specificity for the peptide.
Since there is no overwhelming production of interfering antibodies
to the carrier, this antiserum can routinely be used without further
purification. Pseud-Immune™ control
immune serum (Cat no. GPA018E) from a mock immunized animal is available
to be used in conjunction with this antibody as well as the immunizing
peptide (Cat no. GPA008N), which can be used to neutralize
immunoreactivity.
Manufacturing
Reference:
Shinagawa,
M., et al. J. Gen. Virol. 67:1745-1750, 1986.
 Polyclonal Anti-human
Polyclonal Anti-human