Ubiquitin
is a 76 amino acid protein that is highly conserved. It is found is all plant and animal species and is produced
in response to stress. The
formation of ubiquitin-protein conjugates is a signal for the selective
degradation of proteins. Ubiquitin
has been immunohistochemically localized to a number of pathological
inclusions. These lesions include Lewy bodies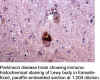 of Parkinson's disease,
neurofibrillary tangles of Alzheimer's disease, Pick bodies of Pick's
disease, Mallory bodies of alcoholic liver disease, cytoplasmic bodies of
a specific myopathy, and Rosenthal fibers within astrocytes.
of Parkinson's disease,
neurofibrillary tangles of Alzheimer's disease, Pick bodies of Pick's
disease, Mallory bodies of alcoholic liver disease, cytoplasmic bodies of
a specific myopathy, and Rosenthal fibers within astrocytes.
A
rabbit antiserum to a purified preparation of human ubiquitin protein is
currently available. This
antiserum has been shown to be immunoreactive  with the purified protein by
ELISA. This antiserun reacts immunohistochemically with ubiquitin-containing
lesions in formalin-fixed, paraffin-embedded tissue. This antiserum is
especially useful for the immunohistochemical identification of Lewy
bodies, which are not well differentiated by histochemical stains. It will
also immunolabel ubiquitin on protein blots.
This antibody should be a valuable tool for scientists working to
understand the role of ubiquitin in various neurological diseases.
with the purified protein by
ELISA. This antiserun reacts immunohistochemically with ubiquitin-containing
lesions in formalin-fixed, paraffin-embedded tissue. This antiserum is
especially useful for the immunohistochemical identification of Lewy
bodies, which are not well differentiated by histochemical stains. It will
also immunolabel ubiquitin on protein blots.
This antibody should be a valuable tool for scientists working to
understand the role of ubiquitin in various neurological diseases.
Manufacturing
Reference:
Sparkman, D.R., et al.
Prep. Biochem. 21:93-104, 1991.
 Polyclonal Anti-human
Polyclonal Anti-human